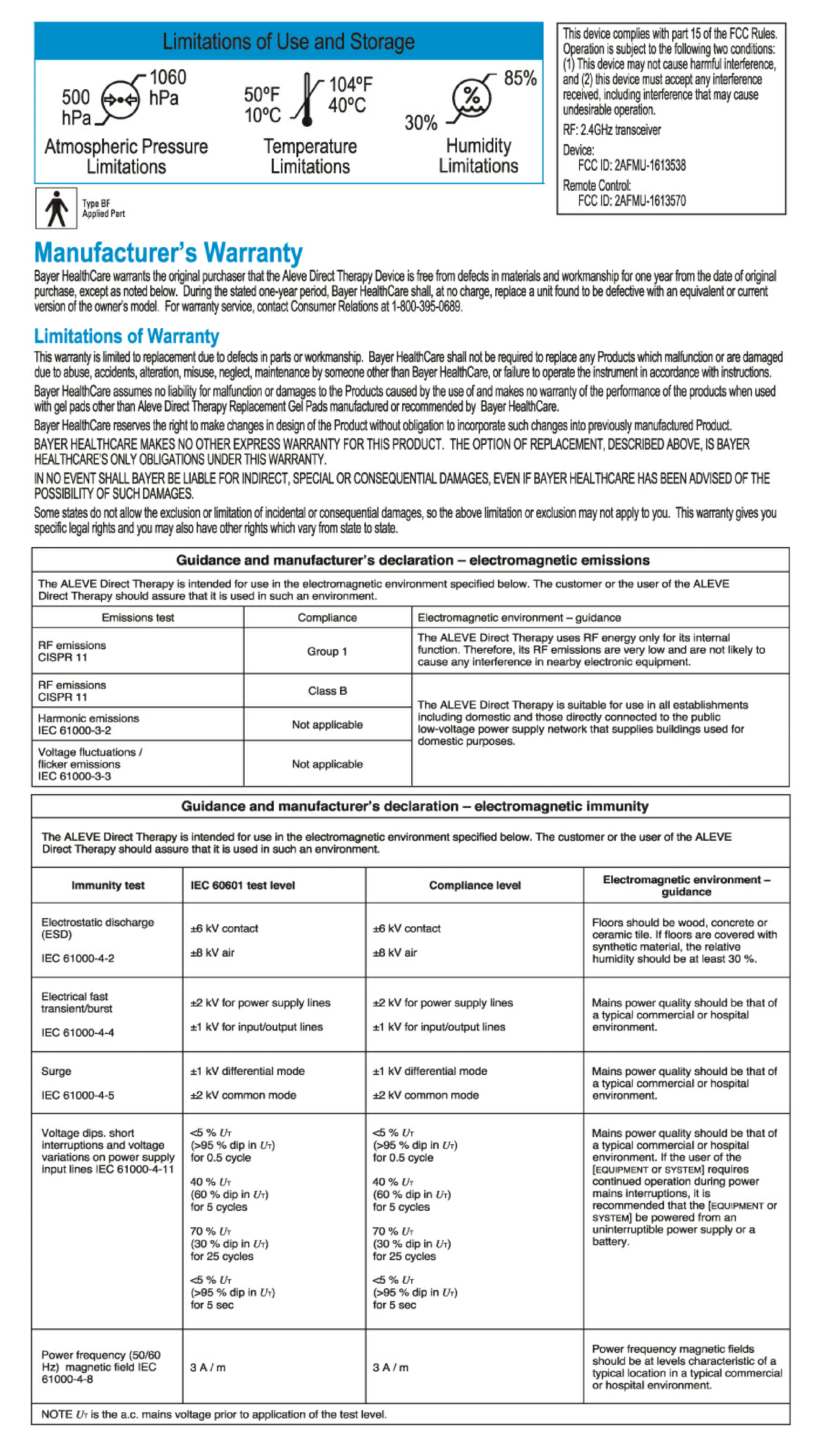
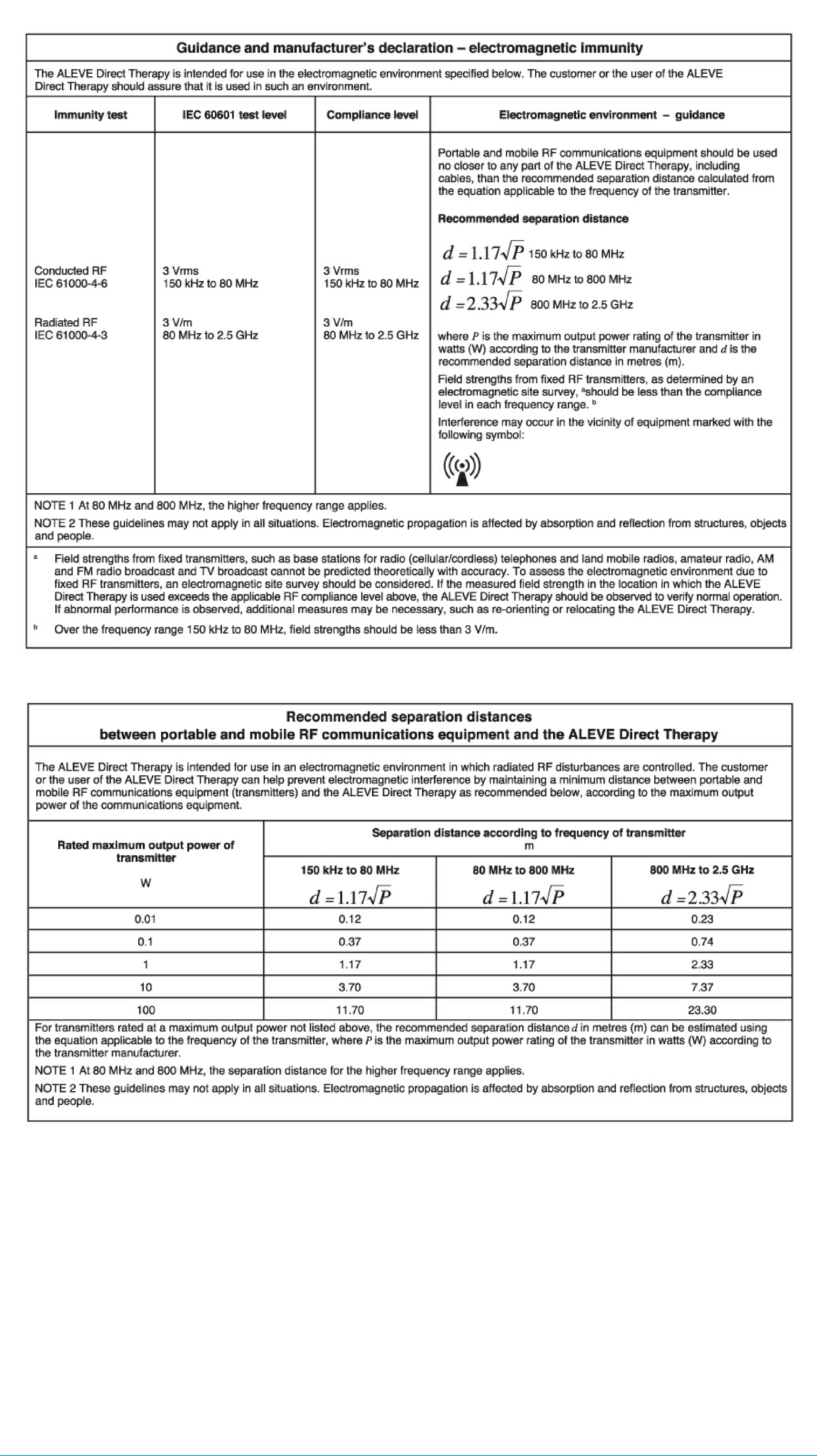
Update 2017-12-18: Bayer now has 2 FCC registrations 2AFMU-1613538 & 2AFMU-1613570
As of 30 May 2017, the FCC ID registration for the Aleve Direct Therapy TENS Device is missing ( FCC ID 2AFMU-1613538 / 2AFMU-1613570 )
There are no registered products under the grantee 2AFMU, Bayer Healthcare LLC
User Manual for Aleve Direct Therapy TENS Device:
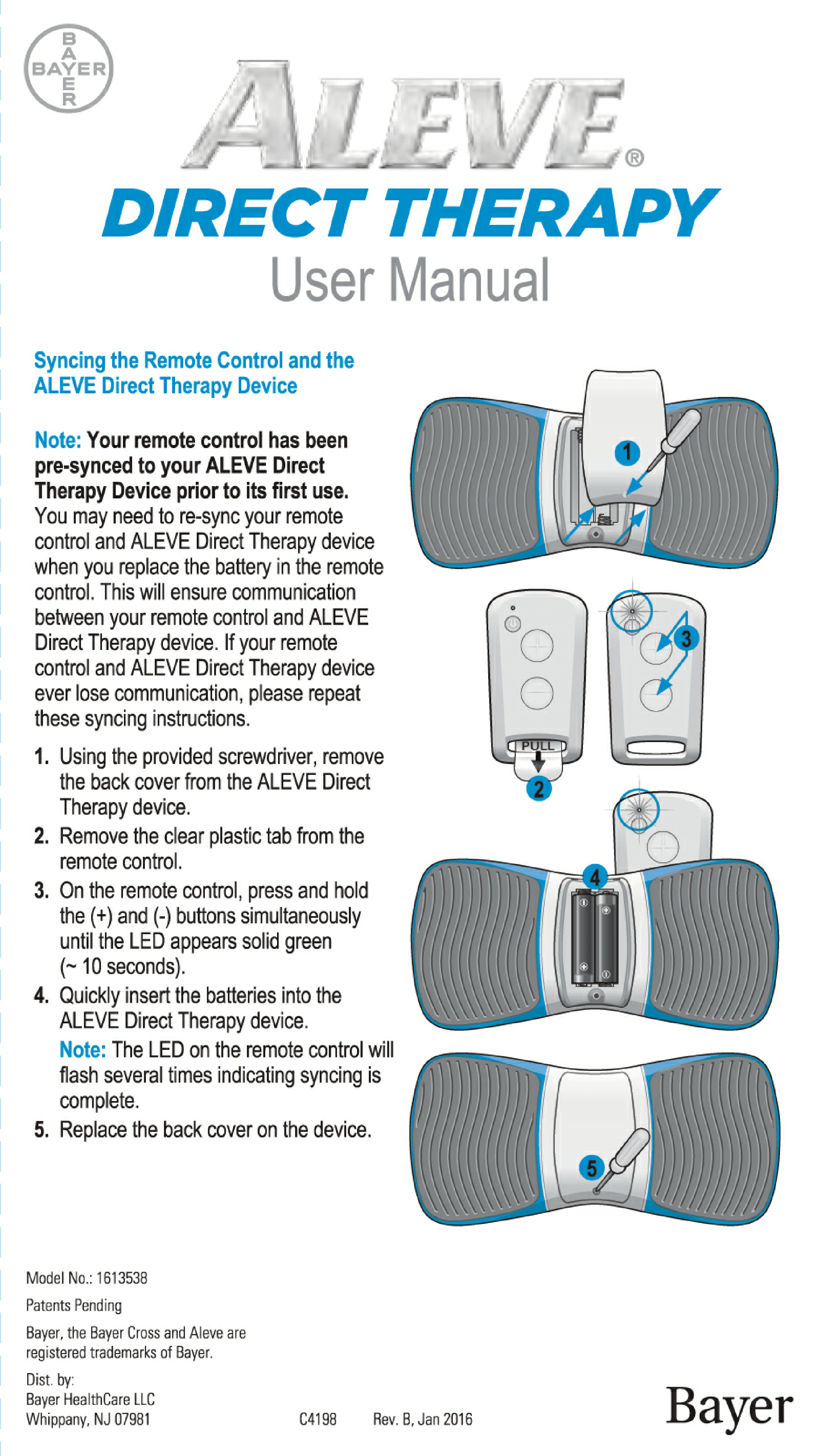

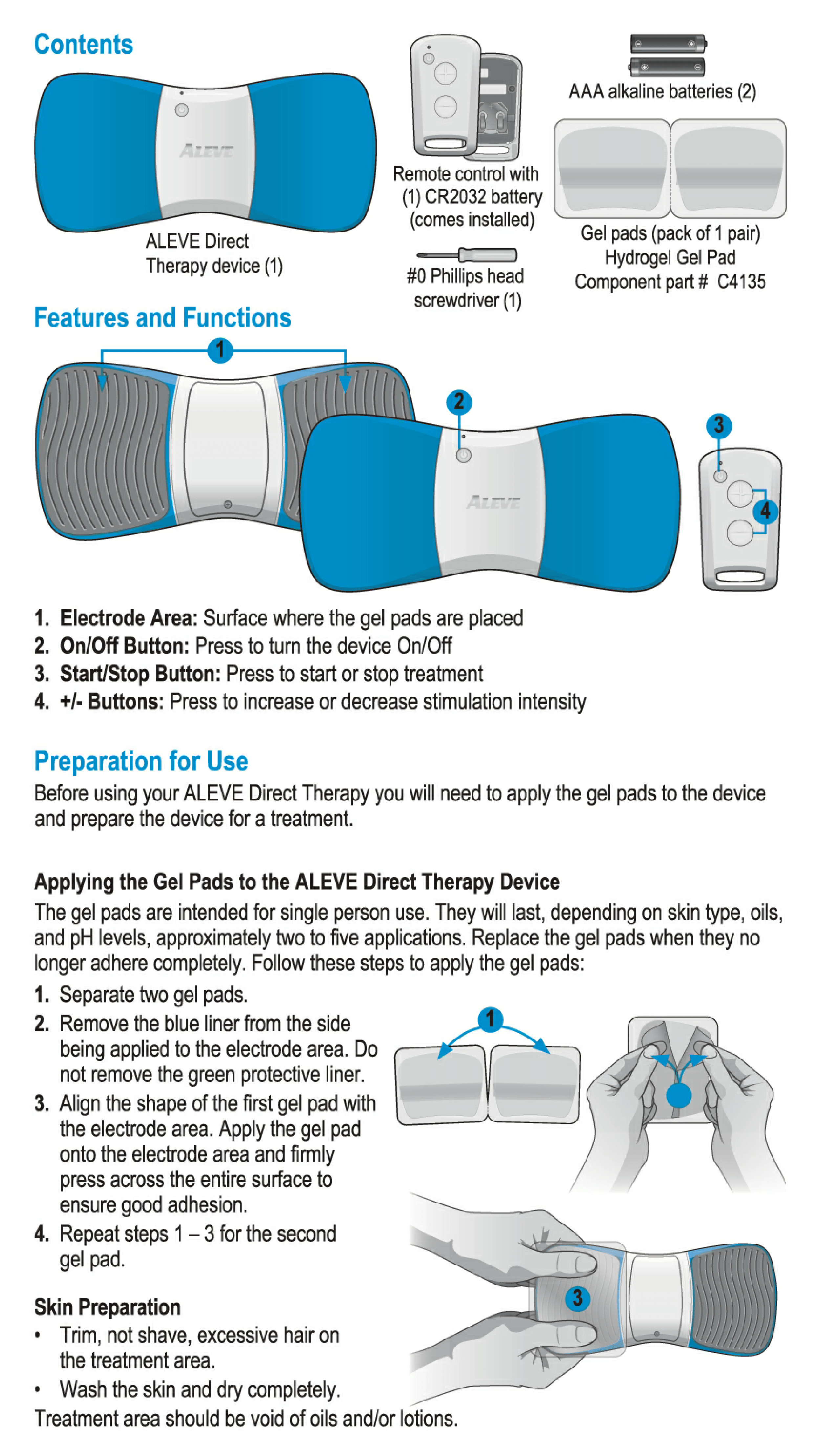

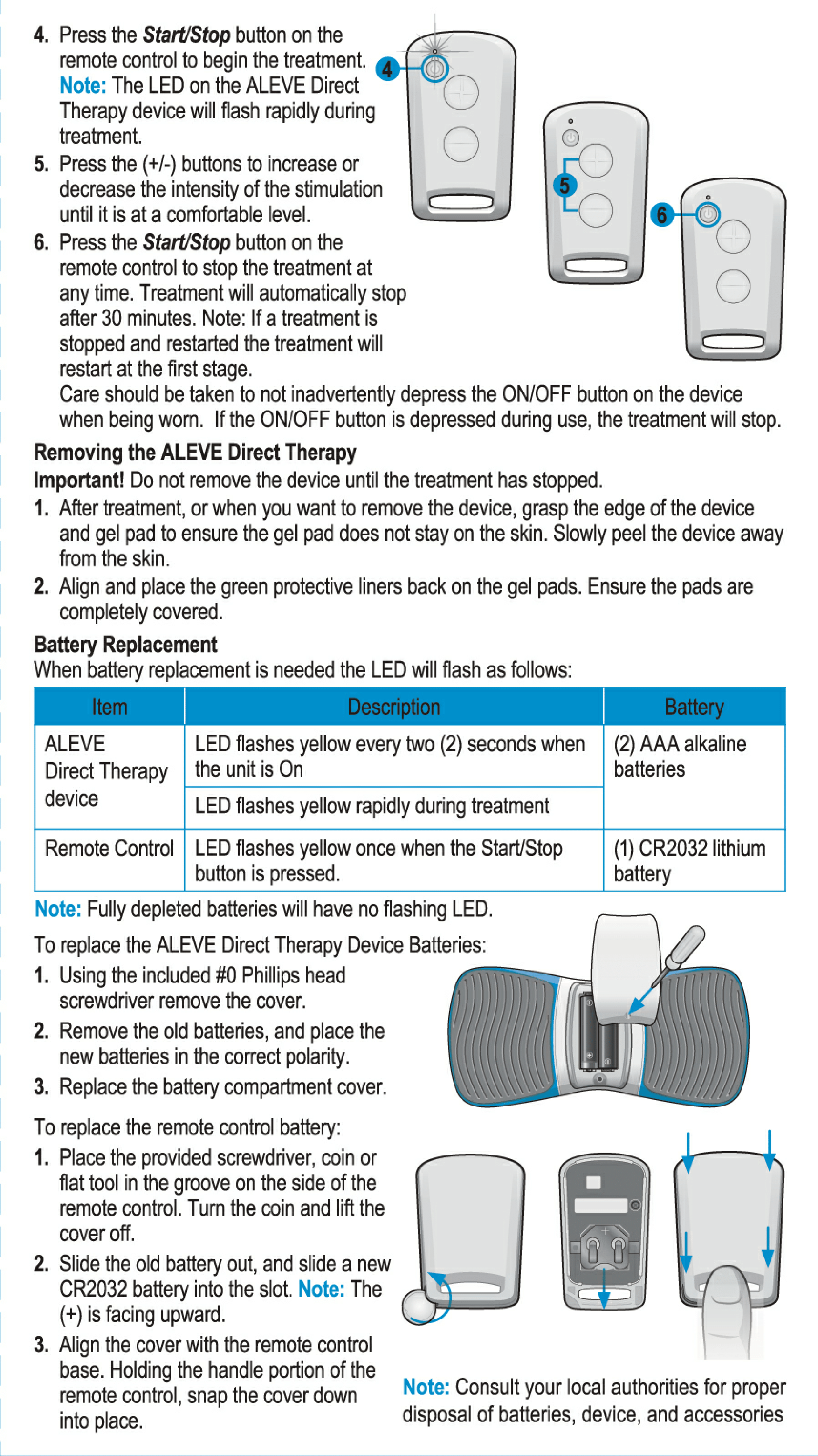
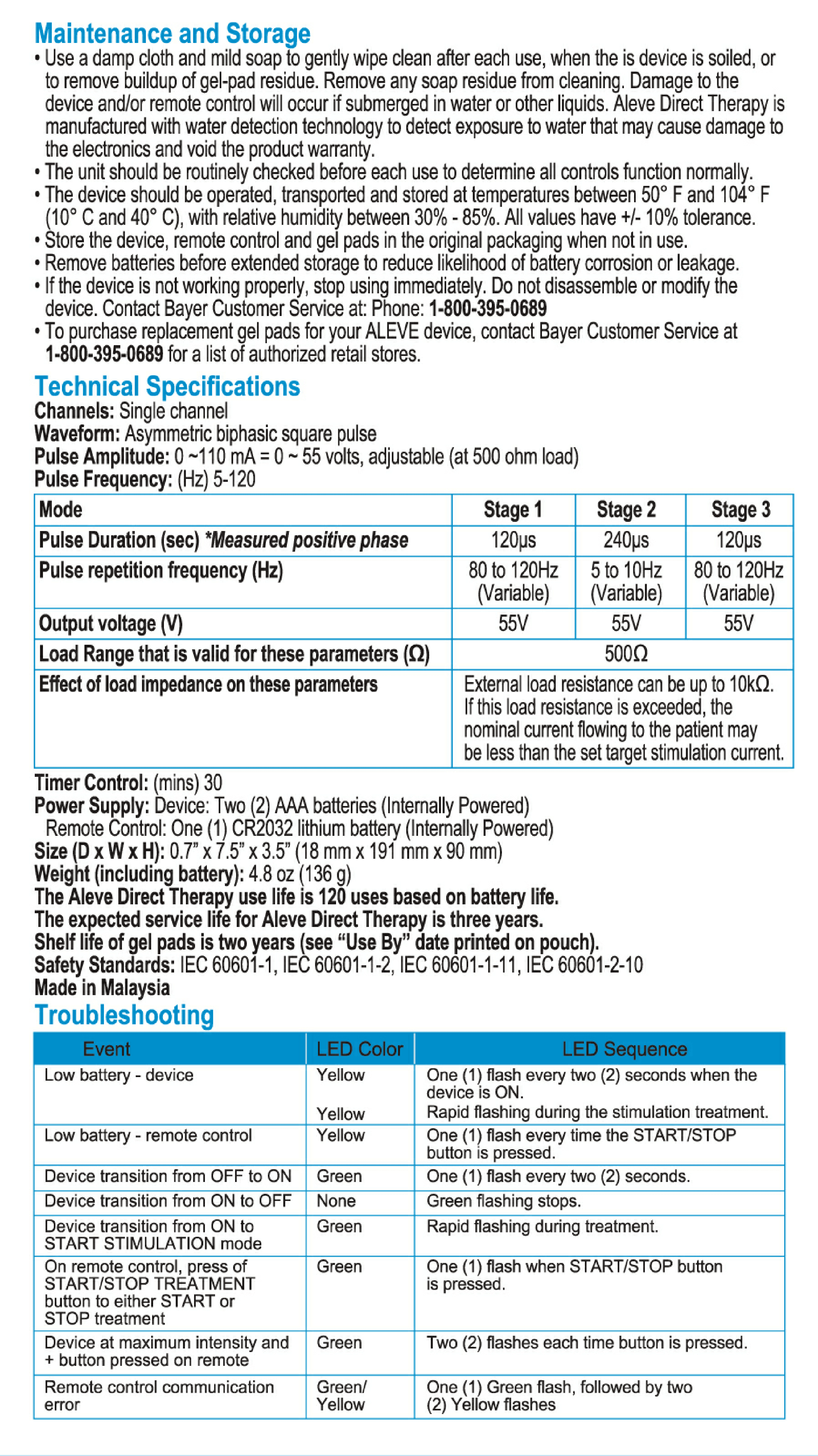
My Aleve direct therapy is not working. I have changed batteries in both the main unit and remote . They both blink green. But there is nothing felt in the the gel pads on my back. I tried re-syncing but not sure the instructions are clear but with no response as manual states.
It was a very effective unit when used last summer. I hate to throw it out. Please advise
as to what i can do.
I need gel pads. I lost the ones that came with it.
The best thing I ever bought to help with my back pain. On the back of the unit where you put the batteries the numb broke off so when you screw the back on it now just falls off. Was very disappointed today when I called the number and they said no longer make the product and can’t get any parts.